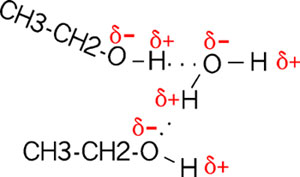We are starting on the problem of how the bacterium E. coli reproduces, how it grows; how we get two E. coli cells from one. But first we need to know what are the chemicals that need to be made if we are to create one net E. coli cell.
::Water - Most abundant single molecule::
Let's start with the most abundant and most important molecule in the cell, not an organic molecule,
but water, H2O. We will use our discussion of the water molecule as a springboard for introducing different types of chemical bonds that are important in biology.
A more accurate representation than H20 would be: HOH, showing that each hydrogen atom is bonded to the oxygen atom. Or H-O-H, where the dashes indicate covalent bonds, the sharing of electrons.
Or, showing the actual angle formed by the 2 bonds to H:
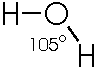
or, showing the sharing of electrons more explicitly:
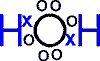
Covalent bonds are very
strong bonds.
We can compare the strength of various chemical bonds by noting the amount
of energy it would take to break the bond. For
most covalent bonds this is about 100 kilocalories
per mole (kcal/mole), where calories are a unit of energy (1 cal
= energy needed to raise 1 gram of water 1 degree C; Calorie in dieting
(with an uppercase C) = kilocalorie (kcal)).
A yet more accurate portrayal would be:

This configuration [Purves6ed
2.8] has important consequences, because although the electrons are
indeed shared between the H's and the O, they are not shared equally.
The oxygen nucleus is more electronegative than the hydrogen nucleus,
that is, it attracts the shared electrons more strongly than the hydrogen
nuclei. As a result, the O is slightly negatively charged and the H's
are slightly positively charged.
The ![]() 's indicate a
partial charge, as opposed to a full charge.
You'd get a full charge if the electron were to be completely captured
by one of the partners, resulting in the formation of charged ions, as
chlorine atom does in table salt (NaCl --> Na+ and Cl-).
's indicate a
partial charge, as opposed to a full charge.
You'd get a full charge if the electron were to be completely captured
by one of the partners, resulting in the formation of charged ions, as
chlorine atom does in table salt (NaCl --> Na+ and Cl-).
So water is a polar molecule (one with a charge separation), and this property has profound consequences for biological molecules.
::Water forms hydrogen bonds::
As a result of this polarity, each water molecule can be attracted to
another water molecule, depending on the orientation. This attraction
is very sensitive to orientation, being sharply maximal when the O - H -
O atoms are lined up:
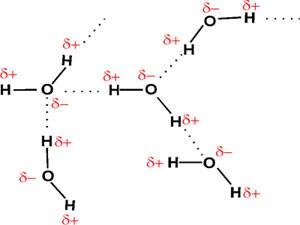
These connections between two molecules are called hydrogen
bonds. Their strength is about 3 kcal/mole, thus they are weak
bonds compared to the strong covalent bonds of ~100 kcal/mole. To chemically
break covalent bonds by the thermal motion induced by heat, you would
typically need hundreds of degrees (e.g., breaking oxygen-oxygen bonds
when burning coal). In contrast, hydrogen bonds are readily disrupted
at temperatures between freezing and boiling (0o - 100oC).
In fact, freezing and boiling of water is a reflection of the hydrogen
bonding: Gas = so much thermal motion that no hydrogen bonding is possible;
liquid = H-bonds are constantly forming, breaking, and re-forming; solid
= hydrogen bonds are locked in a stable crystal structure, which is ice
[Purves6ed
2.15] (see another
ice).
Are there H-bonds in compounds other than water? Sure. Consider ethanol (alcohol), which has an hydroxyl group (-OH, see functional groups handout; we will be discussing almost all of these functional groups at one time or other). Compare CH3-CH2-OH, vs. ethane (CH3-CH3) which does not have this polar hydroxyl group. The hydroxyl group is polar, for the same reason as in water. So it can H-bond to water when it is in an aqueous solution (as most biological molecules are). It is for this reason that most compounds with polar groups are very soluble in water. That is, they are constantly forming thse weak bonds to the water molecules.
Note that carbon always forms 4 bonds.
And the H-bonds are not limited to oxygen in O-H groups: nitrogen is also more electronegative than hydrogen, as in an amide (-CO-NH2), and oxygen is more electronegative than carbon (as in the carbonyl in the same amide):
("R" is shorthand for any general organic group, one that is not necessarily
relevant for the discussion at hand.)
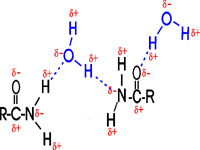
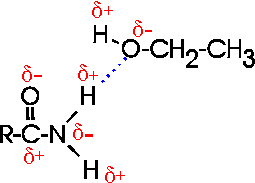
How about H-bonds between organic molecules? Sure, if they can find each other: e.g., ethanol-acetamide, and the orientation is important here, as with water. (If the amide in the diagram were acetamide, the R would be CH3.) [Purves6ed 2.9] In aqueous solutions such interactions will always be competing with water molecules, which are usually more abundant....
::Water molecular structure
used as a springboard to discuss all weak bonds::
Having introduced the subject of weak bonds,
I want to now complete the discussion of bonds by introducing all of the
bonds that play important roles in the behavior of biological molecules.
There are five:
Chemical bonds (5):
covalent (strong)
hydrogen (weak)
ionic (~weak)
hydrophobic (weak)
van der Waals (weak)
| Covalent | Hydrogen | Ionic | Van der Waals | Hydrophobic forces |
| ~100 kcal/mole | ~3 kcal/mole | ~ 5 kcal/mole | ~1 kcal/mole | ~3 kcal/mole |
| electrons shared | water-water | full charge transfer | fluctuating | not a bond per se |
| organic-water | can attract H-bond | induced dipole | entropy driven | |
| organic-organic | strong in dry crystal | at close range only | only works in water | |
| strong | weak, orientation sensitive | weak in water | weak | weak |
1. covalent bonds: electrons shared, strong [bond energy of ~ 100 kcal/mole] = energy needed to pull the 2 bonding atoms apart]
2. hydrogen bonds:
water-water [~2-3 kcal/mole]
organic molecule - water
organic - organic molecule
orientation-dependent
3. Ionic bonds: Full charge transfer; NaCl = strong in the dry crystal (need a hammer to break it)
But ionic bonds are weak in water. Why? Water can H-bond to the charged
ions: Na+ and Cl-. This process is called solvation [Purves6ed
2.11].
So is this bond between water and the ion an H-bond or ionic bond? Half and half. Maybe 5
kcal/mole.
--- 3A. Organic molecules can form ions too [acids
and bases]:
::Organic acids and bases::
ACIDS: molecules that are able to lose a proton (hydrogen ion) easily, such as a carboxyl group (a
carboxylic acid):
R-CO-OH ---> R-COO- (net charge = -1), + H+.
BASES: molecules that are able to take up a proton
easily (protons being always around to some extent in water [ e.g., at
10-7M at pH7]), such as amines:
R-NH2 + H+ --> R-NH3+
Carboxylic acid will be the only organic acid and amines will be the only
organic base we will discuss this semester.
Acidity and basicity are measured by pH (= -log[H+]) [Purves6ed 2.18].
Under the right conditions, ionic bonds can form between two organic ions, with a bond strength of about 5 kcal/mole (in water):

Where are we going with all this chemistry, and these weak bonds? We started describing the molecules of E. coli, with the idea that we have to know what we have, in order to know what we have to make, to replicate an E. coli cell. The weak bonds I am cataloging for you now show how these molecules can interact - but as we proceed to consider larger and larger molecules, they will help us to understand the structure of the individual large molecules, such as proteins and DNA. So this is more than just a listing, the weak bonds will be very important, as we will see in the next few lectures.
4. Van der Waals bonds: Exist between any 2 molecules
Only effective at very close range (e.g., 0.1 nm, or 1A).
Fluctuating induced dipole.
~1 kcal/mole.
These are the weakest of the bonds we'll discuss, about 1 kcal/mole, but they are able to form between any two molecules. Van der Waals interactions form between fluctuating induced dipoles. Take for example two methyl groups, where the C and H have about the same electronegativity, so there is no intrinsic charge separation. A momentary negative charge can develop in the electron distribution around one of these atoms, and this charge will induce the opposite charge in a nearby atom's electron cloud. These bonds are only effective at extremely short range (~ "touching"). Indeed, the size of an atom in space is often estimated by its "van der Waals radius." (Closest approach before repulsion between nuclei sets in).
5. Hydrophobic interactions ("bonds")
Not really bonds, but often referred to as such.
Caused by the effects of water on the association of other molecules.
Non-polar (apolar) molecules are unable to form H-bonds with water.
E.g., octane, CH3-(CH2)6-CH3. (~= gasoline)
Water molecules surrounding an apolar molecule take on a relatively ordered
structure compared to the constantly switching H-bonding patterns made
with other water molecules.
This ordered cage structure is minimized by interfacing
the apolar molecules with each other:
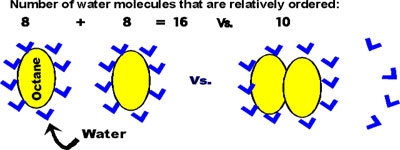
when aggregated, the increase in disorder of the water molecules that become
freed from the cage structure is so great that the entropy of the system
is greater with the octane molecules coalesced. This increase in entropy
provides a hydrophobic force equivalent to about 2-3 kcal/mole (per mole
of octane, in this case).
The actual bonds between the octane molecules in a coalesced glob in water are just the van der
Waals bonds.
This state of affairs is not intuitively obvious.
The bottom line is that apolar groups will tend to associate with other
apolar groups in aqueous solutions. Click here for an alternate view of
hydrophobic interactions.
[methane]
[watercage]
This finishes our introduction to the chemical bonds
that will be important in our consideration of biological molecules.
Let's get back to the chemical make-up of an E. coli cell...... Water was molecule #1.
::Macromolecules vs. small
molecules::
High molecular weight vs. low molecular weight
We have about 5000 more different types to consider. Before proceeding to #2, let's place all
molecules into 2 classes:
1) small; and 2) large.
Small ~< 500 daltons, or molecular weight units), corresponding to about 50 atoms; Large ~> 5000
daltons (~500 atoms).
These size distinctions are not sharp boundaries, just a rough gauge.
The large molecules are usually called macromolecules. The small molecules are just called
small molecules.
Small = e.g., a molecule like propylene, a synthetic organic chemical: CH3-CH=CH2. What is a large version of this small molecule? It is not like the picture below (where each circle represents propylene):
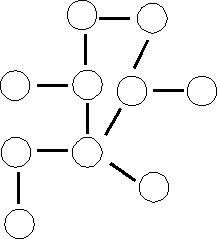
Rather, polypropylene, a familiar plastic, is a linear polymer of propylene:
O-O-O-O-O-O-O-O-O-O-O-.
::Polymers vs. monomers::
Virtually all of the biological macromolecules are built this same way:
they are linear polymers of small molecules. This simplifies matters
greatly.
Nomenclature: Monomers --> di-mers (two small molecules linked
together covalently) --> tri-mers --> tetramers, etc. ƒ.--> oligo-mers
(moderate numbers of repeating units), --> poly-mers (lots of
repeating units).
The monomers could be all the same, as in certain polysaccharides
like cellulose (glucose (n)) or they could be different, the extreme
example being proteins, where there is a mixture of 20 different
monomers present.
While this greatly simplifies our consideration of these large molecules,
there is plenty of complexity left.
Many of the important small molecules in the cell are these monomers,
the basic building blocks of the biological polymers. The polymers, or
macromolecules, comprise 4 classes:
-polysaccharides,
-lipids,
-nucleic acids, and
-proteins.
The total number of such monomers is about 40..... Pretty simple...
Now there are about a dozen or so other small
molecules that serve other functions (they are not monomers that will
end up in polymers). These are co-factors that are important in the catalysis
of chemical reactions in the cells. So this brings us to ~50 different
small molecules so far.
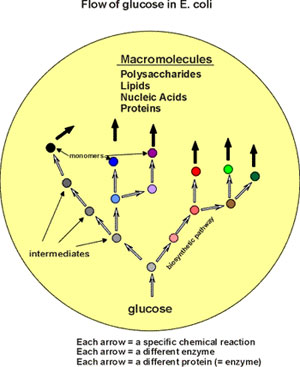
Then, necessary but less generally important, are the "intermediates".
All the carbon in E. coli can flow from glucose via biochemical pathways
(see flow handout - p.13 - for overview)
This is what glucose looks like:
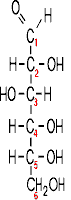
For instance, this is what one of the monomers of a protein looks like:
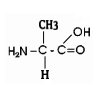
These molecules are quite different. In the cell,
a molecule of glucose is converted, in a series if chemical transformations
into the product, (here, alanine), a monomer for building proteins. Intermediate
types of molecules are created along the way on this pathway (a metabolic
pathway). In general,
glucose --> A --> B --> C --> D --> E --> monomer --> polymer
[A, B, C, D, and E here are the intermediates.]
These pathways are of various lengths. If we take 10 as a generous estimate of the intermediate steps in an average pathway, then we get another 450 (i.e., 9 x 50) different small molecules to add to our total in the E. coli cells. So our final number of small molecules is about 500. Not too great a number to master. Most are known. We will get to know the majority of the end-products, the monomers, as well as a few of the intermediates.
We will continue our discussion of the molecules
of E.coli by focusing on the polymers, the monomers being considered in
the context of the macromolecules of which they are a part.
A simple overview of the kinds of molecules in
the cell, then, is [Handout
figure 2-7]:
(C) Copyright 2001 Lawrence Chasin and Deborah
Mowshowitz Department of Biological Sciences Columbia University
New York, NY
Pictures referred to are from Purves, et. al., Life, 5th Edition,
Sinauer-Freeman's Images of Life 5.0.
A production of the Columbia
Center for New Media Teaching and Learning