::POLYSACCHARIDES::
(Monomers are sugars; sugars are carbohydrates)
So, first, POLYSACCHARIDES: The MONOMERS here are
SUGARS. Most common is glucose (also called dextrose). Let's look at the
structure of this hexose (a sugar with 6 carbon atoms):
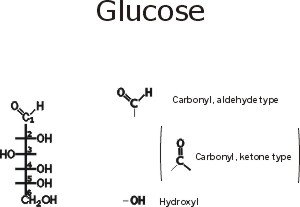
::Glucose::
Note the functional groups here: a carbonyl (aldehyde or ketone) and a bunch of hydroxyls. Is it hydrophilic or hydrophobic? And so is it very soluble in water? (yes, like sucrose, table sugar). Note the numbering system, so we can talk about the various carbons in the chain. Numbering usually begins at the end closest to the carbon that has the least number of hydrogens or most oxygens. Sugars are carbohydrates, with a general formula of CnH2nOn; the term refers to carbon compounds with many hydroxyls and a carbonyl (C=O) group.
Compare the diagram drawn here with that in the middle of the glucose handout to note some organic chemistry shorthand. Since carbon is so common in organic compounds and always takes four bonds, we can simply leave it out, with the understanding that a carbon atom is present at the vertex of 2 or more bonds. Similarly, we can adopt the convention of leaving out the hydrogens, which form only a single bond: thus a bond line with nothing appended to it means that there is a hydrogen there.
::Ring formation::
In 3-dimensional space, a hexose chain can easily curl up, such that the oxygen attached to carbon 5 can be juxtaposed next to carbon 1. A 6-membered ring forms preferentially in water, by attack of the hydroxyl of carbon-5 (C5) on the carbonyl double bond at C1. One bond of the carbonyl double bond opens up and forms a new bond between carbon-1 with the O of C5. The H leaves C5 and a new OH group is formed on carbon 1. Follow along with the diagram on the glucose handout, So a 6-membered ring is formed, with O as one of its members (one of the vertices). One carbon (C6) is left sticking out away from the ring. Unlike most biochemical reactions, which require a catalyst to help them take place at a reasonable rate (more on this in a week or so), this intramolecular cyclization reaction takes place all on its own, as soon as a sugar is put into a water (aqueous) solution. The ring structure can also open up, re-forming the straight chain. The 2 forms are in a dynamic equilibrium, but because the ring form is more stable, this species predominates in water.
::Anomers: alpha and beta glucose::
Now, when the O of C5 approaches the C1 carbonyl double bond, it can do so from one side or the other side. Depending on which side is attacked, the resulting ring comes out looking different in 3-dimensional-space. That is, the resulting ring can be of two different conformations in space. The two conformations are formed at about equal frequencies. The 2 conformations are called alpha and beta:
Alpha, where the C1 OH that is formed ends up on the same side of the ring as the -OH of C2, or Beta, where the C1 OH that is formed ends up on the opposite side of the ring from the -OH of C2: see glucose handout, right side. See also a picture [Purves6ed 3.11].
In sugars, carbonyl carbons that can switch the side of their hydroxyl groups when cyclized are called anomeric carbons, and the two resulting sugars (alpha and beta forms) are called anomers. See sugars [Purves6ed 3.11], and more sugars [Purves6ed 3.12a], [Purves6ed 3.12b]
::Chair form::
The ring is actually not flat, but puckered into a reclining chair-like shape, but hard to draw: (see flat vs. puckered) - - -in this chair-view the hydrogens and the hydroxyls can be seen to be not really up or down, but rather either axial (vertical, sticking up OR down) or equatorial (horizontal, sticking out).
Note in glucose all the hydroxyls are equatorial except that of the #1 carbon in the alpha conformation. In beta-glucose this -OH is upper, relative to the hydrogen, and equatorial; in alpha-glucose it is lower (relative to the hydrogen) and axial. These conformations in space have important consequences as we shall now see as we consider the polysaccharides formed by glucose monomers.
::Glycosidic bonds::
As we consider a polymer built from glucose monomers, we can first consider a dimer. Two glucose monomers can be connected to form a DIMER. This connection, WHICH DOES NOT HAPPEN BY ITSELF (i.e., without some help from a catalyst), involves a dehydration, the removal of one molecule of water, from the 2 monomers:
2 monomers ----------------------> dimer
R-OH + R-OH ---------> R-O-R + HOH
This type of reaction is also referred to as a CONDENSATION, as it condenses two molecules into one.
The resulting -C-O-C- bond is called a glycosidic bond when it is connecting two sugars.
Conversely, the breakdown of polymers back to their constituent monomers involves the reversal of this chemistry, the addition of water, or hydrolysis (the products = a hydrolysate).
R-O-R + HOH ------> R-OH + R-OH
Both of these reactions require different catalysts in the cell in order to occur, which is generally true for all the biochemical reactions we will discuss. See the Carbohydrates handout, below the line for a depiction of two dimers in the flat ring forms. Note the 1-4 linkage (C6 sticks out of the ring, so that is one way to figure out the numbering in the ring). Although the bonds are presented as bent at right angles, they are not really so, it is just a way of presenting both sugar monomers right side up and still connect them with a glycosidic bond.
There are several different hexoses in most cells. Fructose, galactose, and mannose are some common ones. Differences lie in the positions of the carbonyl along the chain and relative positions of the hydroxyls in space. Fructose has a ketone carbonyl at C2, and cyclizes to form a 5-membered ring (still with one member oxygen, of course, so 2 C's stick out from the ring (Carbohydrates handout).
And there are several common disaccharides (see Becker: p.64):
Glucose-glucose via a 1-4 alpha-link is maltose, where alpha refers to the state of the -OH in the monomer joined at its C1 carbon [Purves6ed 3.13a]. Maltose is formed as you digest bread.
Glucose + galactose via a 1-4 beta-link is lactose (in milk) [Purves6ed 3.12b],
Glucose + fructose via a 1-2 alpha-alpha link = sucrose (table sugar). [Purves6ed 3.12b]
But these are not yet polymers, or macromolecules.
These dehydrations can continue in many cases in a repeated way to form chains that contains 1000's of monomers:
X--1,4--X--1,4--X--1,4--X--.........(where X represents a sugar ring)
::Starch and cellulose: function follows form::
A poly-glucose of this type is CELLULOSE, which contains exclusively glucose molecules in beta linkages The beta linkage results in has a pretty straight connection between the C1 and C4 of adjoining carbon atoms, since they both are equatorial and so are sticking out. Thus a cellulose chain extends straight with its C6 OHs sticking out from the chain on either side. [Purves6ed 3.14a1] Many cellulose molecules can then associate side by side (via hydrogen-bonds to each other) to form a fiber of great strength (e.g., in cotton, and it also contributes to rigidity of wood ). [TINKER TOY demo]. Cellulose is the most abundant carbon compound in the biosphere, accounting for about half of all such carbon.
 |
If glucose molecules are put together with an alpha 1,4
link instead of beta, then a polymer of a different shape results.
Here, the C1 -OH is axial whereas the C4 -OH in glucose is (always
is) equatorial. The angle of this alpha 1,4 bond is such that the
polymer bends at each glycosidic connecting bond. As a result, it
takes on a helical shape that again allows lots of hydrogen bonding
between glucoses in each turn of the helix, thus stabilizing the polymer
in this shape. [TINKER TOY demo].
Such is the case with STARCH, which consists of alpha-glucose molecules joined in 1,4 linkages. In addition, starch has branches made [Purves6ed 3.14b] by linking additional glucose molecules at the C6 OH of some of the glucose residues in the chain, via an alpha 1,6 bond). The branch continues with alpha 1,4 linkages (see Becker: Fig. 3-24). The length and frequency of the side chains give rise to the different forms of starch (potatoes, corn) or of a starch like polymer found in mammalian liver, GLYCOGEN ([Purves6ed 3.14a2]). These polymers act as storage forms for glucose. When glucose is needed, they can be hydrolyzed (adding water back to the bond between the monomers) to regenerate the free monomer. {Q&A}. |
Here is our first good example of an important theme in biochemistry, the relationship between structure and function at the molecular level. The straight linear structure of cellulose made possible by the beta-linkages allows the assembly of thousands of aligned molecules to produce a cellulose fiber of great tensile strength. The alpha-linkage in starch produces a compact structure, not strong, which serves as a storehouse of glucose for energy when needed.
Your texts have additional examples of important polysaccharides. Some of the sugars have nitrogen-containing groups appended to the basic carbohydrate ring. The rigid bacterial cell wall is another example, like cellulose, of a polysaccharide used for structural support. So is the shell, or exoskeleton, of insects (CHITIN). [Purves6ed 3.15c]
::LIPIDS: Soluble in organic solvents::
LIPIDS: This is a more heterogeneous group, being defined as substances in a cell that are extractable in organic solvents. Non-polar compounds are not soluble in water, as they tend to coalesce. But they ARE soluble in non-polar solvents such as benzene (a hydrocarbon - compounds made up of just hydrogen and carbon atoms, like the octane molecule we considered earlier). So lipids are molecules that have extensive non-polar regions. Steroids such as cholesterol and testosterone have multiple hydrocarbon rings, and are in this category [Purves6ed 3.24] (see Becker: p.72). Note the drawing conventions, with further shorthand: the depiction (not) of C's and H's. C is assumed to always have 4 bonds; unless otherwise specified, C's are assumed to be present at the vertices on drawn bonds; bonds from such C's that are not shown are assumed to be to H, to make a total of 4. Almost no atoms are named, yet the structure is completely defined.
::Steroids::
Steroids are small molecules that are not monomers that become connected to form polymers. Cholesterol is a component of the cell membrane, which we discuss in a few minutes. Steroids such as testosterone, estrogen, cortisone, and vitamin D are hormones, compounds that circulate in the blood to send signals from cells in one part of the body into cells in another region. You will learn more about steroids in the physiology section in the second semester.
::Fats: Fatty acids and glycerol as monomers::
A major class of lipids are the fatty acids, long straight chain hydrocarbons with a carboxyl group (carboxylic acid) on one end. See [Purves6ed 3.19], and another picture. {Q&A}.
::Esters; fats vs. oils; saturated vs. unsaturated fats::
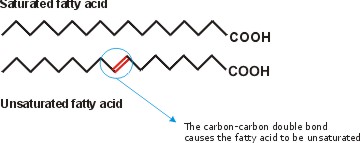
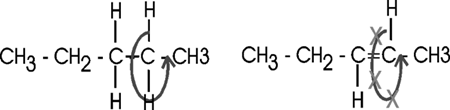
Inside cells, fatty acids (FA) are usually connected to a molecule of the tri-hydroxy (tri-alcohol) compound glycerol. Once again water is removed, this time producing an ester bond (acid + alcohol, draw, see top right corner of lipids handout). If all 3 OH 's on the glycerol are substituted with FA's, then we have a triglyceride. See [Purves6ed 3.19], and another picture. This is fat. You can also have mono- or di-substituted glycerol. Fats differ according to the exact nature of the FA's that are present. "Saturated" fats have -CH2- (methylene) groups, usually 18-20, along the chain. They are saturated with hydrogens, compared to the unsaturated variety, that may have a double bond or two within the chain, and thus have less H's (unsaturated). The presence of the double bond puts a crimp into the structure (unlike single C-C bonds, there is no rotation about C=C double bonds), [Purves6ed 3.20] so it is more difficult for the fatty acid molecules to associate. Thus, unsaturated fats (WITH the double bonds) are usually liquids (oils) while their saturated counterparts (with NO double bonds) aggregate into solid fat.
Take vegetable oil (unsaturated), and add hydrogen across the double bonds and you get Crisco, or the creamy texture in peanut butter (read the label: hydrogenated).
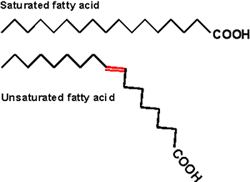
So here again as in the case of polysaccharides, the 3-dimensional structure of the molecule has a lot to do with its physical properties.
Fats are a good example of hydrophobic forces at work. Just think of a fatty chicken soup with those globules of fat floating on top, out of solution.
Fats serve as a storage form of energy. That is, like glycogen or starch, fats can be broken down and used for energy metabolism, as we will see later. Fats are stored in cells called adipocytes.
::Phospholipids, phosphoesters, phosphatidylcholine::
There is a special class of lipids that are related to the fats, but with a significant difference. These are the phospholipids, an example of which is shown in the middle of the LIPIDS handout. Two of the glycerol hydroxyls are connected to long chain fatty acids, but the third is connected to quite a different group, a phosphate. Phosphoric acid (H3PO4) is an acid, The -OH groups attached to the phosphorous easily lose hydrogens at neutral pH. It has 3 acidic hydrogens. [If you are shaky on pH, ask to review it in recitation section.]
The phosphate group is connected to a glycerol hydroxyl, again by a dehydration that forms an ester (acid + alcohol). Whereas we had a carboxylic acid ester linking the fatty acid to the glycerol here we have a phosphoester. In both cases the ester is formed by an alcohol linked to an acid. After linkage, the phosphate group is is still charged, as shown. The phosphate may be free, as in a phosphatidic acid, or it may be esterified to yet another alcohol via another of its acidic groups; a common one is ethanolamine: HO-CH2-CH2-NH3+. The resulting phospholipid would be called phosphatidyl-ethanolamine.
::Phospholipid bilayer: membranes::
Phosphatidyl-ethanolamine is a compound that is highly hydrophobic throughout most of the molecule, but then has a highly polar group at one end, with two complete, if opposite, charges. A further derivative has 3 methyl (-CH3) groups bonded to the nitrogen instead of H's. This moiety is choline (tri-methyl-ethanolamine); the nitrogen retains its positive charge. When esterified to a diglyceride one gets phosphatidyl choline [Purves6ed 3.21]. The polar end can interact strongly with water (it is hydrophilic), while the remainder of the molecule wants to come out of aqueous solution. This is a confused molecule. What happens is that the hydrophobic parts all line up with each other to minimize their interface with water, while the charged ends remain in contact with water. See [Purves6ed 3.22], and a photo. It is in this way that biological membranes form, as a phospholipid bilayer, the charged ends of the double layer being on the outside in contact with water, with the cytoplasm on one side and the exterior of the cell on the other side: See picture.
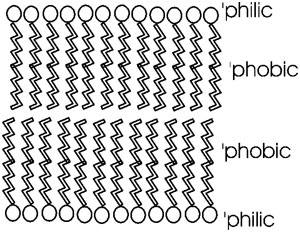
Such a bilayer presents a permeability barrier to water-soluble compounds, which cannot pass through the hydrophobic barrier. Special structures that are embedded in this membrane are then necessary to allow the passage of water soluble compounds in and out of the cell. These are the channels and pumps mentioned earlier. See picture of some membranes in a cell [Purves6ed 5.1].
Large amounts of cholesterol are embedded in the membranes of animal cells. The cholesterol is kept inside by hydrophobic forces. It acts to plug spaces that could cause leakiness, to impart more strength, and to prevent too much association of the saturated fatty acids at low temperature (i.e., "freezing" of the membrane into fat).
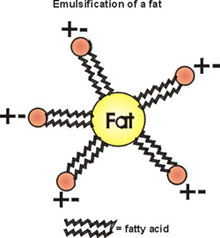
Another property of phospholipids is in emulsification. Lecithin, high in egg yolk, is the old name for phosphatidylcholine. It can be used as a way to break fat globules down to smaller physical units (but not breaking covalent bonds) by associating with the fat with its hydrophobic tails, while leaving the polar groups on the outside to facilitate solubilization in water (e.g., in the bloodstream).
The texts have nice diagrams of all this (e.g., B: 25-26, 180-181).
Lipids are impressive in their variety (see picture) and especially in membrane formation, but admittedly they are not really good examples of the linear biopolymers that we defined. But they have to go somewhere, and so they are stuck amongst the macromolecules.
(C) Copyright 2001 Lawrence Chasin and Deborah Mowshowitz Department of Biological Sciences Columbia University New York, NY
Referred pictures are Purves, et. al., Life, 5th Edition, Sinauer-Freeman's Images of Life 5.0.
A production of the Columbia Center for New Media, Teaching, and Learning