![]() Biology
C2005 Lecture 8
Biology
C2005 Lecture 8
::Free energy,
delta G and delta Go::
We now have some idea of how the rate of chemical
reactions in the cell are speeded up so that they can occur in time frames
consistent with cell growth. But what about this problem of DIRECTION,
a problem the catalysts cannot address. Are all the
chemical transformations in these biosynthetic paths, for example, exergonic,
so that the direction of carbon flow from glucose is a spontaneous reaction?
The answer is no, quite the opposite. Most of the chemical transformations
needed in a biosynthetic pathway are by themselves endergonic, and will
not go from left to right unless something is done about it. Thus
the building of a new E. coli cell must deal with this energy problem,
and much of the cell machinery has indeed been devoted to the solution.
To understand the problem, we must discuss it in some more quantitative
detail, and for this purpose, the use of the concept of FREE ENERGY CHANGE
associated with a chemical reaction is very useful.
We can define a unit of
energy such that it is tied to the directionality of a chemical reaction
as follows:

For the model reaction A + B <--> C + D, written in the left-to-right
direction indicated:
IF Delta G IS <0 THEN A AND B WILL TEND TO PRODUCE C AND D.
IF Delta G IS >0 THEN C AND D WILL TEND TO PRODUCE A AND B.
IF Delta G IS 0 THEN THE REACTION WILL BE AT EQUILIBRIUM: NOT TENDING
TO GO IN EITHER DIRECTION IN A NET WAY.
In an energy diagram:
Note that the Delta G is independent of the route between the starting
reactants and the final products (say, 3 kcal/mole, for all 4 routes shown
here).
Free energy is that part of the energy change associated
with a chemical reaction that can be harnessed to perform work.
We can measure Delta G according to the following
equation [must take this on faith here]:
Delta G = Delta Go + RTln([C][D]/[A][B])
where A, B, C and D are the concentrations of the reactants and the products
AT THE MOMENT BEING CONSIDERED.
This ratio is sometimes called Q (so Delta G = Delta
Go + RTlnQ).. Q is NOT the equilibrium constant, though it looks
like it...:
Here are the terms:
R = UNIVERSAL GAS CONSTANT = 1.98 CAL / DEG K MOLE
T = ABSOLUTE TEMP. (0o C = 273o K; Room temp = 22o
C = 298o K)
ln = NATURAL LOG
Delta Go = A constant, a QUANTITY RELATED TO THE INTRINSIC
PROPERTIES OF A, B, C, AND D. {Q&A}
The first term, delta Go, relates to the QUALITY of the reactants and products, and the second term,
RTlnQ, relates to the QUANTITY of the reactants
and products, how much of each is present.
What is this Delta Go? It is called
the: STANDARD FREE ENERGY CHANGE of a reaction.
One useful way to define of it is to consider the special case when all
the reactants and all the products are present at 1 unit concentration.
The change in free energy of this reaction is:
Delta G = Delta Go + RTln(1), or Delta G = Delta Go
+RT x 0,
or delta G = delta Go (a special case when all participants are at 1M)
So Delta Go is
the free energy change that occurs when all participants in the chemical
reaction are at unit concentration. What is unit concentration? 1 M, for
most reactants and products (water and hydrogen ions are treated differently,
as we'll see. )
One way to think of this delta Go
is to picture 1000 moles of A, B, C and D in 1000 liters of solution.
So all components are at 1M. One mole of A + B is now converted to C +D.
The energy absorbed is the standard free energy
change. Note that the concentration of reactants has not appreciably changed
in this hypothetical condition.
Why make such a fanciful and arbitrary situation?
By defining the conditions for a STANDARD
free energy change, all reactions can be compared to one another, regardless
of the particular conditions; it puts them all on an equal footing. The
Delta Go reflects the nature of
the reactants and products without regard to their concentration. Note
that the actual free energy change of a reaction
does depend on these concentrations, and the
SECOND TERM of the equation for delta G takes these into account. {Q&A}
Please note from the above explanation that delta G and delta Go
are not the same thing. That distinction is important.
::Equilibrium::
Delta Go can be calculated, although not accurately, from tables
of free energies of formation from simple atoms or molecules, comparing
these values for the reactants and the products. But it is most easily
and accurately determined experimentally, by measuring the concentration
of the reactants and products at equilibrium. For at equilibrium, Delta
G = 0 and
Q = Keq, (a special case). So: Delta Go = -RTlnKeq.
If we measure A, B, C and D at equilibrium, let's say we get: [C]eq[D]eq/[A]eq[B]eq
=
2.5 x 10-3
Note very little C and D are formed from A
and B in this case.
Then Delta Go = -2 x 300 x ln (0.0025) = -600 x -6 = 3600
So the calculated Delta Go is +3600 cal/mole, or +3.6 kcal/mole.
So 3.6 kcal will be absorbed when 1 mole of A + B goes to C + D. Energy
is absorbed rather than released, confirming the lack of a tendency of
C and D to be formed from A and B.
That is, since the standard free energy change
is positive, this reaction does not tend to go to the right, but rather
to the left. That is, if we start with 1M A, B, C and D, then C + D -->
A + B. So A and B will build up at the expense of C + D.
Note:
If we write A + B --> C + D, Delta Go = +3.6 kcal/mole, then
we can also write:
C + D --> A + B , Delta Go = -3.6 kcal/mole (the equilibrium
constant for this C+D -->
A+B reaction is just 1/2.5 x10-3, or 400)
Note also, that if the reactants combine in ratios other than 1:1, we
can write:
aA + bB <--> cC + dD, Delta G = Delta Go + RT ln [C]c[D]d
[A]a[B]b
::Summary
of free energy changes::
And to reiterate: the free energy change of an overall reaction is independent
of the route taken by the reactants, it could be direct or indirect [see
energy diagram with routes that go up or down to intermediates].
The reaction will go to the right if delta G is <0 and to the left if
delta G is >0. At delta G = 0 the reaction is at equilibrium, and under
this special condition (delta G = 0) Q is equal to the Keq.
We stated that delta
Go is called the STANDARD free energy change and represents
the free energy change when these reactants and products are observed
to react with each being at a concentration of 1M, which is the standard
condition, by definition. However, biochemists make 2 exceptions to the
definition of these STANDARD CONDITIONS in the cases of water and hydrogen
ion concentration. Since these two components in a reaction are usually
constant in a biological reaction, they are defined
to be equal to 1 for the purpose of Delta Go calculation when
[H2O]=55M (pure water) and [H+] = 10-7 M (i.e.,
pH7, or neutrality). Strictly speaking, one should acknowledge this use
of these 2 exceptions by designating the free energy change as delta Go'
rather than delta Go, but we will not bother to do that here.
All
chemical reactions have a Delta Go associated with them, a
value which can be written down in a book. Thus any two reactions can
be compared under the standard condition of unit concentration.
The equilibrium constant of a reaction can be easily measured by measuring
the concentration of all reactants and products after the reaction has
reached equilibrium. From the equilibrium constant, one can calculate
the Delta Go of the reaction, since at equilibrium, delta G
= 0, and so Delta Go = -RTlnKeq.
Whereas the first
term in the equation for delta G takes into account the nature of the
reactants, the second term deals with the amounts of the reactants and
products in the situation under consideration. Thus if you know what you
have and how much of each, you can predict the direction in which the
net reaction will go.
::ATP
hydrolysis::
To start our consideration of the free energy changes that are associated
with biochemical reactions, let's consider one of the most important and
fundamental energy-related biochemical reaction, the hydrolysis of adenosine
triphosphate, or ATP. Hydrolysis means the breakage of bonds by the addition
of water. Here:
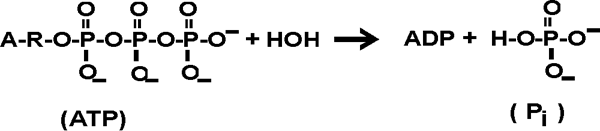
where A = adenine (a base); R = ribose, a 5C sugar; joined to 3 phosphate
groups, which are themselves joined together. See [Purves6ed
6.8].
The Delta Go of this reaction is about -7 kcal/mole.
::"High
energy" bonds ("~")::
Most HYDROLYSIS reactions in the cell do release
free energy, but usually less than 5 kcal/mole. The few, like ATP hydrolysis,
that release more than 5 kcal/mole, are important, because this amount
of energy can be harnessed for useful work, as we'll see. The bonds whose
HYDROLYSIS IS 7 KCAL/MOLE OR MORE ARE CALLED "HIGH
ENERGY BONDS" for this reason. These bonds are not stronger than
other bonds, so this is somewhat of a misnomer, but it turns out to be
a convenient term. It is denoted by a SQUIGGLE when we are talking about
it: AR-P-P~P. Looking at the structure of ATP we can rationalize the high
energy release by seeing that the addition of water relieves the electrical
repulsion between the negatively charged (acidic) phosphate groups. By
such reasoning we would predict that the bond between the first and second
phosphates should also be a high energy bond, and indeed it is, so:
AR-P~P~P. That is, hydrolyzing between P atoms 1 and 2 also releases about
7 kcal/mole (i.e., delta Go = -7kcal/mole)
On the other hand the bond between the ribose and the first phosphate
is not a high energy bond.
Given the Delta Go, one can calculate the equilibrium constant,
which turns out to be about 10+5.
(-7 = RT x 2.3 log K = 0.6 x 2.3 log K; K =10(-7/-1.4)= 105).
You should practice these types of calculations by doing the problems
in Problem Set 4.
(In doing these problems, note that the universal gas constant is usually
given as 1.98 cal/deg-mole, whereas Delta Go and delta G are
usually expressed in kilocalories, so you usually have to divide the RT
term by 1000 to get an answer in kcal.)
I will just note that the concentration of WATER is
not taken into account, as it is present at
55 M in aqueous solution and does not CHANGE in aqueous reactions, and
by convention is defined as 1. (We can make such arbitrary definitions
because we are only considering changes here
[concentration changes, free energy changes], not absolute values.)
So if we start with 1M ATP we will end up with only about 10-5
M ATP remaining at equilibrium.
Yet a 1 ml. solution of ATP will last for days on this bench top. We still
must add a catalyst to get this reaction to go in a reasonable time.
So we add an enzyme, say a pure preparation of ATP-ase
(note nomenclature).
Now all the ATP is hydrolyzed in a few minutes. The reaction has been
allowed to reach its equilibrium and has gone far to the right, because
of the very favorable (large and negative) delta G.
And 7 kcal of energy, free energy, is released. Free energy, so it could
be used for useful work, but what work did it do here? Nothing? Energy
had to go somewhere. It is released as HEAT. The test tube solution warmed
by about 7oC, by my calculation [since 1 ml of solution here,
at 1 M].
This hydrolysis of ATP is in fact the most common reaction the cell uses
to produce usable energy. The trick is to harness this energy chemically,
to put it to useful chemical work. Keep in mind the overall problem, to
make a new E. coli cell that requires many endergonic transformations.
::Coupled
reactions::
So let's take an example of one of these endergonic energy-requiring reactions:
One such reaction is the very first transformation that a glucose molecule
undergoes upon entering an E. coli cell:
+ Pi --> glucose-6-P + H2O; Delta Go
= +3.6 kcal/mole.
(Note that Pi is used as an abbreviation for PO4---)
So the Delta Go is unfavorable in the very first reaction in
the utilization of glucose in the cell.
How bad is the situation?
G + Pi --> G-6-P, Keq = [G6P]/[G][Pi]
If [G] and [Pi] are ~ 0.01M (typical) and Keq (from
Delta Go of +3.6) = 2.5 X 10-3 then [G6P] = 2.5 x 10-7, which is even
below the Km's of most enzymes (for example, for the next reaction taking
G6P further). Now let's consider putting the two reactions we've talked
about together: ATP + H2O --> ADP + Pi Delta Go
= -7 kcal/mole
Glucose + Pi --> G6P + H2O Delta Go = +3.6 kcal/mole
-----------------------------------------------------------------------------
Glucose + ATP --> G6P + ADP Delta Go = -3.4 kcal/mole overall
= net sum of the two considered reactions
So let's mix the reactants together and hope for the best.... I guess
we should add a couple of enzymes to catalyze these 2 reactions: say:
"glucose phosphorylase" and "phosphatase".... But under these conditions
the 7 kcal/mole produced by the hydrolysis of ATP is lost mainly to heating
the surroundings (H2O). And besides, in mammals, where E. coli
lives, the temperature is kept constant, so we cannot influence reactions
by heat. The problem is solved by an enzyme, hexokinase.
This enzyme binds both ATP AND glucose. The
very phosphate from the ATP is transferred
to the glucose molecule. So the overall reaction written here on the bottom
line is not merely the net sum of the two reactions written above it,
rather, it IS the reaction catalyzed by hexokinase.
The phosphorylation of glucose has been COUPLED
to the hydrolysis of ATP. {Q&A}
What about the G6P produced? Does it now contain the high energy phosphate
bond? Well, what is the Delta Go for G6P hydrolysis? Easy:
-3.6 kcal/mole, since reverse reaction (second line) is +3.6. But -3.6
falls short of the -5 needed to qualify for a high energy bond, so no
squiggle here. Remember, if you write a reaction backwards, you just reverse
the sign of the Delta Go.
This coupling to ATP hydrolysis
is a very common way the cell uses to drive otherwise endergonic reactions.
[Purves6ed
6.9] Since the ATP is so often used to "pay for" the energy cost of
these chemical transformations, ATP is called the energy
"CURRENCY" of the cell. Coupling to ATP is one of two ways the
cell manages to run endergonic reactions; we will discuss the second way
a little later.

So is this the solution for E. coli growing on minimal medium? Far from
it; we have just passed the buck. Where's this ATP coming from? Not from
the medium, where glucose is the only carbon source. ATP must be synthesized
from glucose just like all other small molecules. And that itself takes
energy. But, once we have some ATP, most of the molecule can be used over
and over again to provide energy for coupled reactions, as long as we
can re-phosphorylate ADP back to ATP. The problem has thus shifted to
this reaction: ADP + Pi --> ATP (Delta Go = +7 kcal/mole).
If we could find a way to do that, we would have solved our energy problem.
Here is where we have some divergence in the unity
of biochemistry: The world is divided mainly into two types of organisms,
those who can run this reaction using the energy derived from sunlight,
the photosynthetic organisms, like plants; and the rest of us, E. coli,
humans, butterflies, who make ATP from ADP by using the energy derived
from the breakdown (catabolism) of carbon
compounds like glucose. Plants are actually also included in this latter
category, since when it's dark they derive energy from glucose catabolism,
as the rest of us do. We will consider this process of glucose-based energy
metabolism in some detail, and not really consider PHOTOSYNTHESIS, for
lack of time. Obviously, photosynthesis is the more basic and essential
process for the planet, since there would be no glucose if it were not
for the plants and their ability to harness solar energy. But photosynthesis
is a bit more complex, so it is not a good place to start.
So, ATP by way of glucose:
The overall plan is (for E. coli growing in air [dissolved oxygen]):
glucose + O2 --> CO2 + H2O, and: ADP
+ Pi --> ATP
First, an overview: 3 parts: In each of these 3 parts
we will be concerned with our goal: to produce ATP's for use in reactions
that require energy.
1) GLYCOLYSIS: in which the 6C glucose is broken down to a 3C compound,
pyruvic acid [glucose (6C) --> pyruvate (3C)].
2) THE KREBS CYCLE, in which the pyruvate is broken down to CO2
[pyruvate --> CO2]
3) The ELECTRON TRANSPORT CHAIN, in which oxygen is taken up and water
is produced in a separate series of reactions [utilization of O2]
I am now going to discuss GLYCOLYSIS in some
detail, for two reasons:
1) It will illustrate the problems and the solutions of energy requirements,
so one aim is to understand energy metabolism.
2) It is a real life detailed example of a typical metabolic pathway,such
as we have been alluding to with all these arrows leading to A's and B's
and C's. (The pathway will be characterized by a series of small changes
between substrate and product at each step)
::Glycolysis::
I'll show the first few reactions with the sugar in open chain form, because
I think it is easier to see what's going on.
1.
a phosphorylation (kinase enzyme)
2. an isomerization
3. phosphorylation
again... note that we have now used 2 ATPs:
rather than generating energy, we are consuming it, so far.
4. hydrolysis
5. isomerization
of dihydroxyacetone phosphate (as we saw in step 2, but in reverse, transforming
the ketone into the aldehyde; and swing it upside down when done: you
have another molecule of glyceraldehyde-3-phosphate): note now we have
2 of everything for each glucose molecule that entered the pathway, this
will be true from this point on.
So, so far, through rxn 5, we've produced 2 molecules of glyceraldehyde-3-phosphate
(GAL-3-P) for each molecule of glucose we used. AND it so far has COST
us 2 ATP's This ATP debt is a loose end that we will have to deal
with sooner or later.....
6.
Step 6 is an oxidation, GAL-3P and another phosphate to 1,3 diphosphoglyceric
acid, (or 1,3-diPGA).
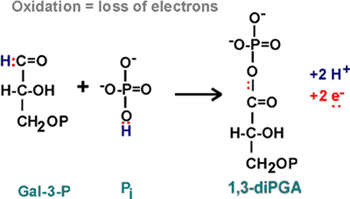
OXIDATION, the loss of ELECTRONS.
We saw it before in the formation of the disulfide bond - there the loss
of two electrons was in the two hydrogen atoms that were taken away. Here,
in reaction 6, we have 2 electrons to be lost from the reactants, GAL-3-P
and phosphate:
::NAD::
Two electrons have been lost. The protons are not important in oxidation,
they are sometimes there with the electrons, and sometimes not. {Q&A}.
These electrons must go somewhere. They are taken up by the OXIDIZING
AGENT which itself will get reduced. As you can see from the NAD
handout, this is a compound called NAD,
or NICOTINAMIDE ADENINE DINUCLEOTIDE [Purves6ed
7.3]:
The nicotinamide part is also the vtramin niacin. Niacin sounds less ominous.
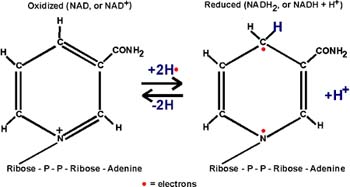
In getting reduced, NAD can accept two electrons, but only one proton.
The other proton goes into solution as a hydrogen ion. But it is the electrons
that are important in oxidations.
Instead of writing the reduction of NAD as NAD+ -->
NADH + H+, we will simply write: NAD --> NADH2, referring to
the two H's that came from glyceraldehyde-3-phosphate, despite the fact
that both proton did not end up on NAD. {Q&A}
This rxn 6 is rather complicated, involving a phosphorylation as well
as an oxidation. The phosphorylation did not require an ATP, but it did
require an NAD.... so now have a new loose end
analogous to the ATP/ADP situation, we have to worry about restoring NADs
from NADH2's as well as ATP's from ADP's.
But at least we have something of value here, a high
energy phosphate, as you can see the squiggle at the top of 1,3-di-PGA
on your glycolysis
handout. That means that enough energy can be released from the hydrolysis
of this phosphate even to drive the phosphorylation of ADP in a coupled
reaction.
Let's take the money and run... this high energy bond is cashed in the
next reaction, rxn 7.
7.
Note that the top carbon is now a carboxylic acid in 3-PGA, whereas it
had been an aldehyde in GAL-3-P. This change is the result of the oxidation
that took place in reaction 6. {Q&A}.
So we've now paid off one of the 2 ATPs the debt we ran up at the start.
Wait a minute, actually, we're all paid up (since we have two di-PGA's
for every mole of glucose that started down the glycolytic pathway).
Continuing along:
8. Isomerization
of 3-PGA to 2-PGA.
9.
Dehydration, water removed. The result is an unstable compound, phospho-enol
pyruvic acid (PEP), one whose hydrolysis can result in the release of
a large packet of free energy.
[2-PGA, as well as PEP, is at a higher energy
level than pyruvate, but the shift in atoms allows
cash-in of ATP along the way, since the phosphate
bond is a high energy bond in PEP.]
10.
Transfer of the phosphate to ADP (X2), resulting in PYRUVIC
ACID (pyruvate), which can be considered the end point of glycolysis.
[Purves6ed
7.7a], [Purves6ed
7.7b], [Purves6ed
7.7c].
So after the 10 steps of glycolysis we have 4 ATPs produced and 2 invested,
for a net gain of 2 ATPs produced from ADP.
So glycolysis does produce energy in the form
of ATP. And the overall reaction will run far to the right, as the Delta
Go from glucose to 2 pyruvates is -18 kcal/mole (even taking
coupled reactions producing the 2 net ATP into account). That is,
1 glucose + 2 ADP + 2 Pi + 2 NAD <--> 2 pyruvate + 2 ATP + 2 NADH2
Delta Go = -18 kcal/mole
The diagram below shows Delta Go's for
the individual steps in glycolysis.
(For a different way of looking at it, see the chart of Delta G's drawn
by Purves [Purves6ed
7.6])

Although the pathway overall is quite favorable, some individual reactions
are quite unfavorable, the most extreme being reaction #4. The energy-requiring
reactions in this case are being pushed by the build-up of reactants by
the more favorable reactions before them, and they are being pulled by
the withdrawal of products by the more exergonic reactions further downstream.
The actual Delta G's will be influenced by the drain of products, so that
the second term in the equation for Delta G is being brought into play
here.
Delta G = Delta Go + RTln([products]/[reactants]),
where RT~0.6 (in kcal/deg-mole)
For instance, if the products are drained such that the ratio of P/R reaches
down to 0.00001, this produces ~ 7 kcal/mole of negative Delta G , enough
to balance out the unfavorable Delta Go of +6.8 for rxn 4.
This indirect affect on the Delta G is the second
method the cell utilizes to carry out an individual reaction that
has an unfavorable Delta Go.
So we have 2 methods:
1.) DIRECT COUPLING of the unfavorable reaction
to an energetically favorable one to produce a new coupled reaction with
a net negative (favorable) Delta Go (as in the hexokinase reaction
#1).
2.) INDIRECTLY, via the WITHDRAWAL
OF PRODUCTS or buildup of substrates
SO, we have our overall negative Delta Go , and we have generated
net ATP, so we should feel pretty good, except for one thing: we have
an important loose end to tie up. We borrowed an NAD to oxidize Gal-3-PO4.
That was a key reaction in the path, as oxidations are usually accompanied
by large changes in free energy. We were able to get a PO4
added to our 3-carbon compound, and this phosphate was the one that was
used to phosphorylate ADP in the very next step (#7). We used NAD, and
it became reduced to NADH2. So now we must consider how we are going to
repay that debt. Otherwise the small stores of NAD in the cell would very
soon all be converted to NADH2 and glycolysis would quickly
grind to a halt.
We need an oxidizing agent to oxidize NADH2. A great one abounds:
O2.
It readily takes up electrons, for instance from Fe++ to make it Fe+++
as steel goes to rust.
::Anaerobiosis::
Does E. coli have access to oxygen? In the lab, yes. We vigorously and
constantly shake the E. coli cultures on mechanical shakers to get air
dissolved in the culture medium to provide a constant source of oxygen,
called an AEROBIC state. In the gut, sometimes yes, sometimes no (crowded).
Do our own cells have access to oxygen? Sure, through the lungs via the
blood vessels to all tissues. But when you are running across campus to
class so as not to miss the first golden words here, your muscles may
need ATP faster than you can deliver oxygen to them for NADH2
oxidation, your muscles will be in an AN-AEROBIC
state. And are many organisms that live in naturally anaerobic environments,
in mud at the bottom of rivers, e.g. So let's first consider the anaerobic
case when no oxygen is available for the oxidation of NADH2.
Under these circumstances the cell must make do with what it has available,
which is mainly: 2 pyruvates. Fortunately, pyruvate itself is able to
act as an oxidizing agent, as seen in rxn 11, where it accepts electrons
into its C=O bond, adding two hydrogens as well, to form lactate, or LACTIC
ACID. It gets the electrons from our NADH2 molecule,
which is really NADH and H+, so it is recapturing one proton (an H+ ion)
from the aqueous pool. Even the Delta Go is favorable, and we get our
NAD regenerated from NADH2. The NAD shuttles back and forth
then, getting reduced in rxn 6 and reoxidized in rxn 11 over and over
and over again, while glucose runs down to lactate, and ADP's are converted
to ATPs to power cell division for E. coli or running up stairs for humans
even in the case of insufficient oxygen.
I think it's the build-up of lactic acid that makes your muscles hurt
if they are doing anaerobic glycolysis too long.
::Fermentation::
So pyruvate is a crossroads: If no oxygen, then if you are E. coli or
humans, you carry out a lactic acid fermentation...
[Purves6ed
7.5b], [Purves6ed
7.15].
If you are yeast, there is a variation on this theme, you break down pyruvate
first to acetaldehyde and CO2, which is not an oxidation, but
then use the acetaldehyde as an oxidizing agent for NADH2,
with the product here being the 2 carbon alcohol, ethanol (rxns 12 and
13). Beer drinkers appreciate this variation, as lactic acid beer would
be pretty bad, and probably not even produce those psycho-pharmacological
effects for which ethanol is famous. {Q&A}.
Just as in the case of ATP, the NAD - NADH2 case is one of re-generation,
not generation. Once a little NAD is made, it can shuttle back and forth
millions of times getting alternately reduced and oxidized:
Consider the efficiency
of fermentation:
For: glucose--> 2 lactates, without considering
the couplings for the formation of ATP's: Delta Go = -45 kcal/mole.
{Q&A}.
Out of this comes 2 ATPs, worth 14 kcal/mol. So the efficiency is about
14/45 = 30%, which is not bad, about what a gasoline engine can do.
Where did the other 31 kcal get to? They are released as heat, which is
why, after you've run fast to class, in addition to the lactic acid PAIN
in your legs, you are also SWEATING.
Now taking the ATPs into account, the overall Delta Go is about
45 - 14 = 31 kcal/mole, so the lactic acid fermentation runs essentially
completely to the right (or clockwise) as written.
::Energy
yield::
The efficiency is pretty good, but on the other hand the YIELD
is poor. What do I mean by YIELD? Well, glucose has a lot more chemical
energy in it than we are tapping here. For example, if we BURN glucose
(i.e., react it with oxygen) and measure the calories of heat given off,
we get no less than 686 kcal/mole. Compare that to the measly 45 kcal/mole
we got from converting glucose to two lactates. The lactic acid that we
are throwing away at the end could be used for more energy, but in the
absence of oxygen there is no way to use it.
(C)
Copyright 2001 Lawrence Chasin and Deborah Mowshowitz Department
of Biological Sciences Columbia University New York, NY
Clickable pictures are from Purves, et. al., Life, 5th Edition,
Sinauer-Freeman's Images of Life 5.0.
A
production of the Columbia
Center for New Media Teaching and Learning