| • | Biosphere 2 Center (Arizona) |
| • | Black Rock Forest (New York) |
Module 11: Chemical and Hydrological Cycles |
Nutrient CyclingBy Dr. James A. Danoff-Burg, Columbia UniversityThe First Law of Thermodynamics states that energy can be transferred and transformed, but it cannot be either created nor destroyed. We can directly apply this principle to ecosystems by following the movement of elements and essential nutrients through the local ecosystem and also on a larger global scale. The study of these cycles is the ecological subdiscipline of biogeochemistry. Vital Elements and NutrientsThe cycles of 18 elements and nutrients are the main ones tracked by biogeochemists. Of these, hydrogen, carbon, oxygen, and nitrogen together account for approximately 96% of the dry weight of plants and as such are important for creating most plant structures. However, all 18 nutrients are essential for life to exist. The other 14 nutrients are called trace essential nutrients are Phosphorus, Sulfur, Potassium, Magnesium, Calcium, Iron, Chlorine, Manganese, Boron, Zinc, Copper, Molybdenum, Sodium, and Silica. The relative abundances of these 18 elements collectively determine how well the local ecosystem functions.
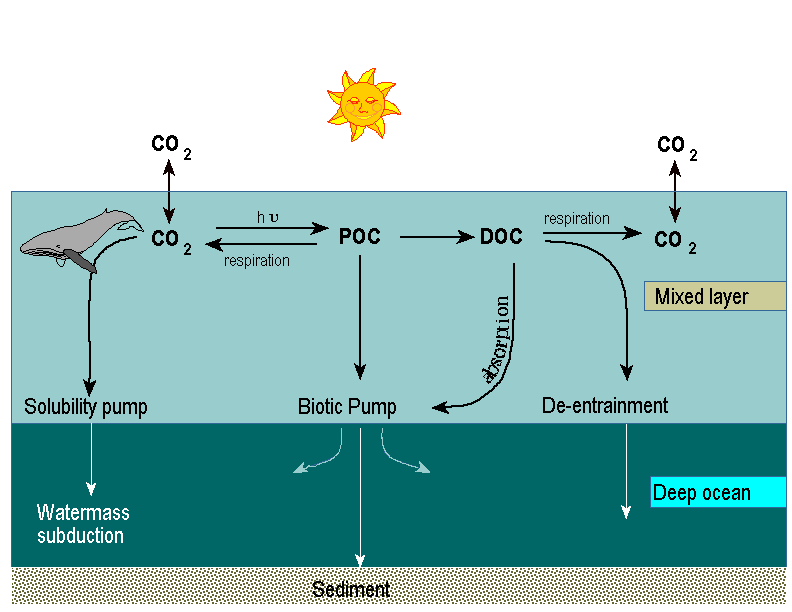
Disrupting the normal proportion of any of these cycles will lead to a concomitant disruption of that ecosystem. The effect of low levels of a nutrient depends on the abundance present relative to others in the ecosystem, rather than the absolute level that is present. Therefore, if you wish to fertilize your lawn, you should not spread only nitrogen or any other single nutrient. Dramatically increasing the concentration of only one element will likely not aid plant growth, given the current species composition, but will instead probably harm it. Deficiencies of these important nutrients and elements can lead to a variety of growth and metabolic abnormalities. Insufficient nitrogen leads to a decrease in protein synthesis and thus a decrease in production of enzymes, amino acids, and other proteins. Lack of any of the 14 trace elements, that are needed in only small amounts, also disrupt growth to the same degree. For example, insufficient iron available to the plant will interfere with the synthesis of chlorophyll and thus inhibit photosynthesis and stop plant growth. This tendency is also taken advantage of when selective plant herbicides are used. Types of Chemical CyclesEach of these elements cycle through the ecosystem by being transmitted between organisms, the atmosphere, and/or the soil in a characteristic fashion. If the element primarily cycles between the soil (or the ocean) and the atmosphere, then it follows the geochemical cycle. If it passes through all three possible components or between organisms and either the atmosphere or soil/ocean then it traces a biogeochemical cycle. Last, if an element cycles between the atmosphere and organisms (for example, phosphorus), then it primarily follows the biochemical cycles (a.k.a. the internal cycle - within organisms only). In truth, all elements utilize the biogeochemical system since they pass through all three components at some stage. The question of which term is most applicable is merely one of relative emphasis. Additionally, there are in the broadest sense 2 components - biological and geological, and both of these can be broken down further. For example, geological components include atmosphere, soil, lithosphere (e.g., ocean sediments storing carbonates, volcanic eruptions creating new fertile terrain), and hydrosphere (ocean mixed and deep layers, lakes, etc). The scale at which each of these three cycles operates varies, in general. Elements that largely undergo geochemical cycles tend to primarily cycle between ecosystems. The ecosystems can be very close to each other, such as an arroyo wash and the surrounding mesa top, or far apart, such as the Sonoran and Chihuahuan desert ecosystems in Arizona. In contrast, biogeochemical cycles are more local and elements cycle through many species within a single ecosystem. Last, an element that primarily runs through a biochemical cycle passes only within plants within an ecosystem. Although these scale generalities are usually correct, there are many exceptions. Geochemical and biogeochemical cycles can cycle at the local or watershed level but also at the global level. Different parts of many biogeochemical cycle can happen continents or oceans away, hence the term "global carbon cycle". For example, the sediments from the Amazon that are rich in organic material are often washed well out into the Atlantic. Similarly, dust storms from the Sahara often add inorganic nutrients to the snowfields of the Alps. Lastly, humans that burn fossil fuels in the wealthy Northern countries add carbon to the atmosphere and enrich plant production globally. Geochemical CyclesThese large-scale transmissions of elements can occur by following either a gaseous or sedimentary cycle. Gaseous geochemical cycles are the main way that nitrogen, carbon, and oxygen cycle through an ecosystem. As we shall see later in the course in Module 15, elevated levels of gaseous oxygenated carbon from combustion engines may be producing the greenhouse effect, which is in turn warming the planet. Only a subset of the important elements primarily undergo gaseous cycles, but all of them pass through sedimentary geochemical cycles. These latter cycles rely on transport via meteorological mechanisms (dust and precipitation), biological mechanisms (animal migrations and human activity), and geological/hydrological mechanisms (geological weathering and erosion). The modes by which elements can be cycled using each of these mechanisms should be self apparent. Each can pick up and disperse elements great distances. By weight, the greatest transportation mechanism of the three for most elements is by weathering. The formation of soil is begun by this mechanism and water transports huge quantities of sediment through an ecosystem. Early during human evolution in the Nile river basin, our ancestors took advantage of the annual deposition of novel, fertile soil for agricultural purposes. Biogeochemical CyclesThis type of nutrient cycle takes advantage of all three possible transmission components: soil, organisms, and atmosphere. The movement of nutrients between the soil and atmosphere occurs mostly because of large scale climatic features. In contrast the link to organisms during biogeochemical cycles is more complex. The link through organisms is of course through plants, but not just through their roots as would be the obvious conclusion. Plant absorption of chemicals is also done through their stomata (e.g., carbon is absorbed primarily through the leaves), and via contact absorption if the nutrient is in solution. Root uptake can occur in three manners—either passive diffusion (during which nutrients move from areas of higher to those of lower concentration); mass transfer (nutrients entering with water absorption; or mycotrophy (e.g., a relationship with a mycorrhizal fungi passing nitrogen that they convert to a useable form [nitrogen fixation] to the roots). Mycotrophy is present in many species of plants and enables the plants to live in areas with poor soil. In nitrogen poor desert habitats, legumes have a similar relationship with a bacterium (Rhizobium spp.) that enables them to be pioneer species in barren areas. Nutrients are lost from plants to the soil and atmosphere during the biogeochemical cycles in four main ways. In wet areas, such as in the Moist Atlantic Forest of Brazil, leaching of the nutrients from the leaves via excess water is a large problem. Herbivory takes nutrients that have been converted to leaves, nectar, pollen, and flowers who then digest and excrete the nutrients to the soil. Mass reproduction disperses plant energy in the form of seeds, pollen, flowers, and fruits to the environment. Last, litterfall serves as the beginning of soil formation. Biochemical CyclesNutrients cycle within organisms as well as within ecosystems, which are generally referred to as either biochemical cycles or internal cycles. Within a plant or an animal, nutrients are regularly removed from one organ for use elsewhere in the organism. Calcium moves to the blood from the bones of lactating mammals. Nitrogen, potassium, and phosphorus are largely translocated from the leaves to the trunk of the tree in the fall before leaves are shed. In this manner trees and other organisms are able to satisfy a significant portion of their annual nutrient requirements using the mechanisms of the biochemical cycle. These internal cycles allow for plant growth in those parts of plants growing on nutrient poor soils to have adequate nutrients via translocation. This also allows plants to recycle nutrients during times of low nutrient availability (e.g., during times of low temperature or moisture). The efficiency of these biochemical cycles, during which nutrients are translocated, and their relative amount varies with the degree of environmental stress. Soils with poor nutrient quality leads to an increased efficiency of translocation in pine trees, relative to nutrient rich soils. Any factor that influences plant growth and plant nutrient uptake can also strongly influence internal cycling. In general, those environmental forces that stress plants will lead to a increased efficiency and a greater amount of nutrients being translocated to necessary tissue. How do all of these cycles influence organismal diversity? The speed and magnitude of many of these cycles influence the level of biodiversity that any given site can support. Those nutrients with large budgets with a short periodicity tend to be able to support a greater level of biodiversity than do those with either small (deserts) or slow (northern tiaga forests) carbon cycles. As has been the case with many of the other abiotic influences that we have been discussing during this Section, chemical cycling helps to set the stage for and makes possible all other ecological activity. Additional Relevant Online ResourcesLycos has a page in their infoplease.com website discussing the Basics of the First Law of Thermodynamics. General Biogeochemistry Resources on the Internet as compiled by Cornell University. Britannica.com has many useful pages on geochemical, biogeochemical cycles in general, and sedimentary geochemical cycles in specific. Put in the appropriate word in the search box to easily search the site. Biochemical cycles are discussed by Dr. Charles Ophardt of Elmhurst College. His discussion also touches upon and slightly blurs the distinction between biochemical and biogeochemical cycles. The Natural Food Hub has a discussion of how grains, beans and seeds were cultivated during early human evolution in the Nile river basin by taking advantage of the seasonal flooding that leads to sudden sedimentary geochemical cycling. A discussion of passive diffusion, mostly emphasizing its relevance to drug absorption across a membrane but is still relevant to its role as a mechanism of the biogeochemical cycle, is available from the Veterinary School at Cornell University. Mass transfer as a mechanism of the biogeochemical cycle, mostly from the perspective of biochemical engineering in porous solids but still relevant, is available from NgeeAnn Polytechnic University. Mycotrophy and mycorrhizal fungi are discussed by PlantHealthCare.com. A general treatment of the theoretical bases of leaching is available from The Chemical Engineers' Resource Page. Translocation as at work within soils and during soil forming processes is available from Dr. Noorallah Juma and Chris Harland of the University of Alberta. All Materials Copyright © 2000 by James Danoff-BurgAll Rights Reserved. |